CYB004

CYB004
Deuterated Dimethyltryptamine (dDMT)
Cybin’s portfolio includes the most advanced and extensive DMT and deuterated DMT (“dDMT”) dataset in the psychedelic drug development sector.
The Company’s CYB004 program leverages significant pre-clinical and clinical datasets from five clinical trials conducted on several molecules: CYB004, SPL028, SPL026 DMT.
CYB004 and SPL028 are dDMT molecules which have both been studied in humans.
SPL026 is non-deuterated DMT, which was part of a robust human clinical trial which showed a large clinical effect in Major Depressive Disorder (7.4 point difference in MADRS between SPL026 and placebo).
CYB004 is currently being evaluated in a Phase 2 study in Generalized Anxiety Disorder (“GAD”). We believe CYB004 is well-positioned to demonstrate a significant therapeutic benefit in the GAD population, as Major Depressive Disorder has significant comorbidity with anxiety: 50% of patients with major depression also have an anxiety disorder.4
Cybin’s internal research has also developed potentially more convenient and patient-friendly dosing methods, including intramuscular (“IM”) dosing, compared to the current intravenous (“IV”) methods used for DMT administration.
Development Status: Initiated Phase 2 proof-of-concept study of CYB004 in Generalized Anxiety Disorder (“GAD”)
Next steps: Phase 2 CYB004 topline safety and efficacy data in GAD expected year end 2024
Intellectual Property
Cybin holds the most extensive deuterated DMT IP portfolio in the sector, with more than 40 patents protecting the dDMT program, including claims directed to composition of matter, methods of use, formulation, and synthesis.
CYB004 is protected by a U.S. composition of matter patent expected to provide coverage until at least 2041.

Target Product Profile
for dDMT optimized with data from 5 clinical studies
To date, Cybin has completed five clinical trials evaluating the safety and
efficacy of CYB004 (dDMT), SPL028 (IM/IV dDMT) and SPL026 (IM/IV DMT), providing key insights informing
the development of the DMT program:
Study
Key Findings
SPL026
DMT IV or IM
(1) Phase 1/2a study in MDD
POC study in moderate to severe MDD (no SSRIs)
(2) Phase 1 SSRI DDI stud
(3) Phase 1 SSRI DDI study
IV DMT is well-tolerated and generates breakthrough psychedelic experience (20-30 min)
Rapid, robust and durable antidepressant effect
46% of MDD patients in remission at 3 months
40% of MDD patients in remission at 6 months
Favorable safety and tolerability in MDD patients
Rapid improvement in anxiety and wellbeing scores
IM DMT is well-tolerated and generates a breakthrough psychedelic experience (~45 min)
Potential enhanced effect when given as adjunctive to SSRIs: 92% remission rate in SSRI cohort vs. 20% remission (non-SSRI cohort)
DMT safe and well-tolerated when co-administered with SSRIs
CYB004
dDMT
(4) Phase 1 Study of IV CYB004 and IV DMT
90 min infusion was well tolerated with strong psychedelic effects at the highest dose
Bolus + infusion provided sustained psychedelic effects over an extended period of time
IV dDMT is well-tolerated and generates breakthrough psychedelic experience (~45 min)
SPL028
dDMT IV or IM
(5) Phase 1 IM/IV study
IM dDMT dose is well-tolerated and generates breakthrough psychedelic experience (average ~90 mi)
SPL026
DMT IV or IM
Study
(1) Phase 1/2a study in MDD
POC study in moderate to severe MDD (no SSRIs)
(2) Phase 1 SSRI DDI stud
(3) Phase 1 SSRI DDI study
Key Findings
IV DMT is well-tolerated and generates breakthrough psychedelic experience (20-30 min)
Rapid, robust and durable antidepressant effect
46% of MDD patients in remission at 3 months
40% of MDD patients in remission at 6 months
Favorable safety and tolerability in MDD patients
Rapid improvement in anxiety and wellbeing scores
IM DMT is well-tolerated and generates a breakthrough psychedelic experience (~45 min)
Potential enhanced effect when given as adjunctive to SSRIs: 92% remission rate in SSRI cohort vs. 20% remission (non-SSRI cohort)
DMT safe and well-tolerated when co-administered with SSRIs
CYB004
dDMT
Study
(4) Phase 1 Study of IV CYB004 and IV DMT
Key Findings
90 min infusion was well tolerated with strong psychedelic effects at the highest dose
Bolus + infusion provided sustained psychedelic effects over an extended period of time
IV dDMT is well-tolerated and generates breakthrough psychedelic experience (~40 min)
SPL028
dDMT IV or IM
Study
(5) Phase 1 IM/IV study
Key Findings
IM dDMT dose is well-tolerated and generates breakthrough psychedelic experience (average ~90 mi)
De-risking the development
of CYB004 in Generalized Anxiety Disorder
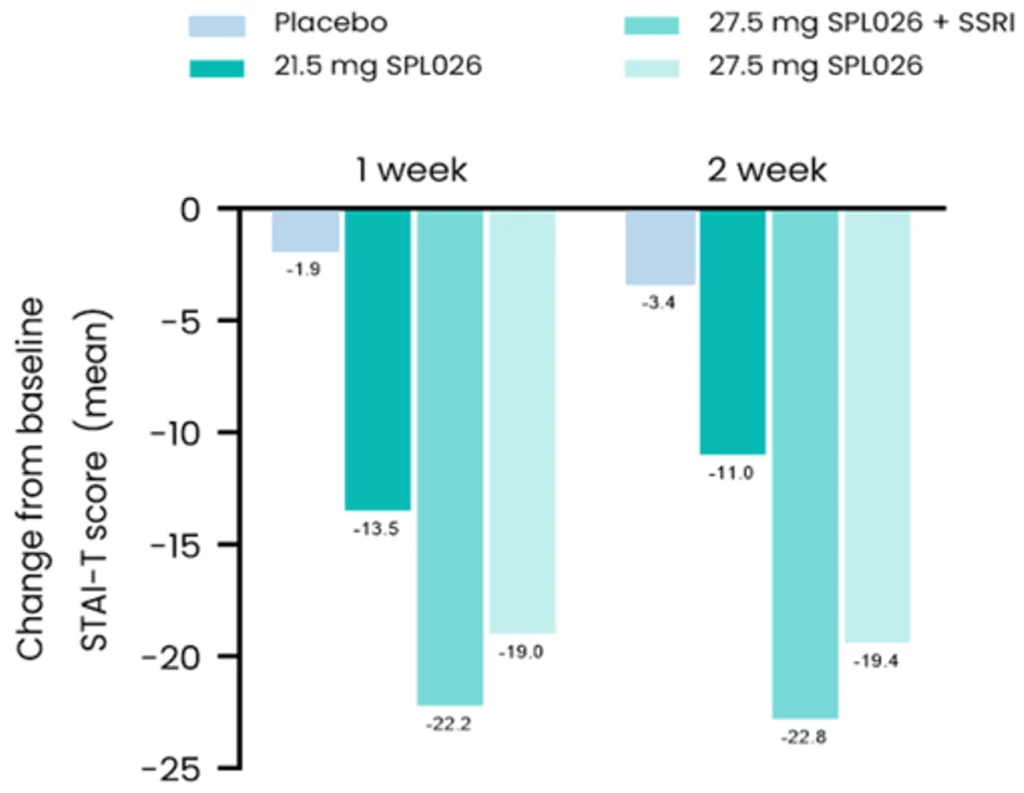
An exploratory analysis of Phase 2a and Phase 1b results for SPL026 (IV DMT) showed significant improvements in measures of anxiety in participants with MDD.
At two weeks, participants on SPL026+SSRIs showed a mean 23-point improvement from baseline on the State Trait Anxiety Inventory – Trait version (STAI-T).
These results suggest a reduction in anxiety symptoms with IV DMT treatment in patients with depression.
This supports the development of DMT/dDMT for the treatment of anxiety disorders, especially as there are high rates of comorbidity between depression and anxiety
Significant Unmet Need
in Generalized Anxiety Disorder

High prevalence and burden of anxiety disorders
Over 300 million people suffer from anxiety disorders worldwide1
GAD is the most common anxiety disorder seen in primary care, with a 12-month prevalence of 2.9% in the U.S.2
Approximately 77% of adults with GAD experience moderate to severe impairment3
High comorbidity with depression: 50% of patients with major depression also have an anxiety disorder4
The current standard of care is limited, and there is an urgent need for improved treatment options
50% of patients with GAD do not respond to first line treatment with antidepressants such as SSRIs and SNRIs2
Only 43% of patients with anxiety adhere to SSRI/SNRI treatment, due to side effects5
Other treatments have significant limitations:
Benzodiazepines: risk of dependence, only recommended for short term use2
Buspirone: delayed onset of effects, adherence challenges with dosing twice daily6
CYB004 has the potential to transform the current standard of care, with a new mechanism of action that could offer improved efficacy vs. existing treatments

Phase 2 CYB004
Proof-of-Concept Study in GAD
Cybin has initiated a Phase 2 study of CYB004, which is designed to evaluate efficacy in GAD, time to onset of effects, and durability of effects to twelve months. This study is being conducted in the United States.
Phase 2 study design:
Randomized, double-blind study in 36 participants with moderate to severe GAD (GAD-7 score ≥10)
Two IM doses, three weeks apart vs. Two low-dose controls
Primary endpoint: change in Hamilton Anxiety Rating Scale (“HAM-A”) score from baseline at 6 weeks following 2nd dose
Topline safety and efficacy data expected in Q4 2024
To learn more about the Company’s pipeline programs and upcoming milestones, please visit the Development Pipeline page.
Sources:
1. Yang et al. (2021). Global, regional and national burden of anxiety disorders from 1990 to 2019: results from the Global Burden of Disease Study 2019. Epidemiology and Psychiatric Sciences 30, e36, 1–11. https://doi.org/10.1017/S2045796021000275/
2. Ansara E. D. (2020). Management of treatment-resistant generalized anxiety disorder. The mental health clinician, 10(6), 326–334. https://doi.org/10.9740/mhc.2020.11.326
3. Kessler et al. (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry, 62(6):617-27.
4. Hirschfeld R. M. (2001). The Comorbidity of Major Depression and Anxiety Disorders: Recognition and Management in Primary Care. Primary care companion to the Journal of clinical psychiatry, 3(6), 244–254. https://doi.org/10.4088/pcc.v03n0609
5. Stein et al. (2006). Antidepressant Adherence and Medical Resource Use Among Managed Care Patients With Anxiety Disorders. Psychiatric Services, 57(5): 673-680.
6. Melaragno, A.J. (2021). Pharmacotherapy for Anxiety Disorders: From First-Line Options to Treatment Resistance. Focus, 19(2): 145-160. https://doi.org/10.1176/appi.focus.20200048




